Data Rich Processing – The Growing Necessity of R&D to GMP Manufacture

Improved diagnosis is driving precision in treatment, accompanied by increasing regulation stringencies from the likes of the MHRA and FDA. This, in turn, ensures processes as well as the products themselves, are fully understood during every step of the manufacturing process.
The requirement for both the R&D and manufacturing processes to be ‘data-rich’ has become a pre-requisite for drug product manufacturers, and purchasers of drug products, alike; a phenomenon driven not only when things go well, but where an irregularity might have occurred, even when confirmed to be within the validated ‘Design Space’ parameters.
Where the products are usually very expensive to manufacture, manufacturers are always keen to use the data to prove, even if an irregularity took place, the batch can still be released rather than simply quarantined and/ or rejected. From a regulatory perspective, the above method of data interpretation and auditing for corroborating a product is safe for release, is also hugely beneficial.
From Theory to Practice:
US based, SP Scientific, has put data necessity at the forefront of its breakthrough suite of new lyo/freeze drying technologies named Line of SightTM, supported in the UK, Ireland, and France, by Biopharma Group.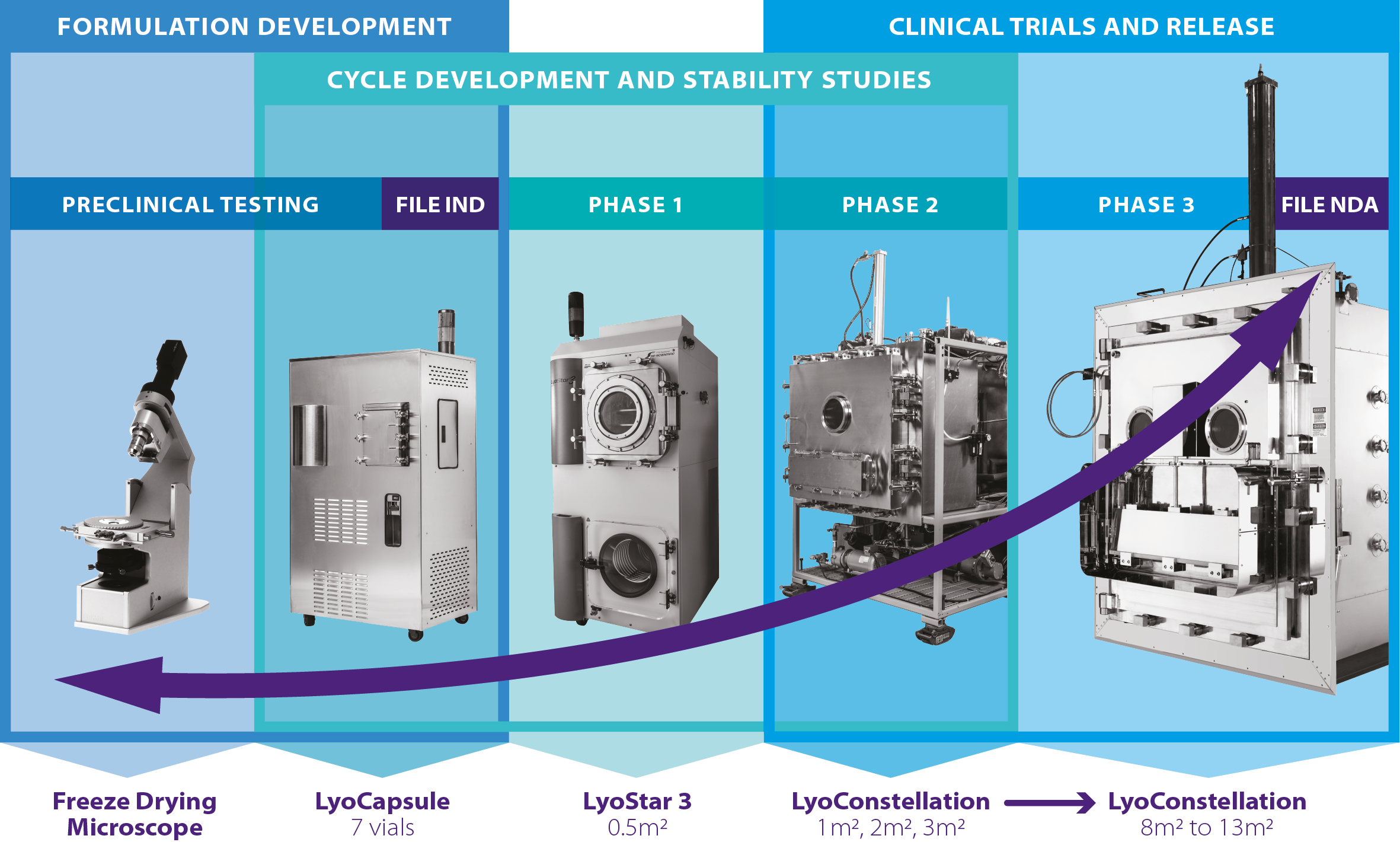
Line of SightTM encompasses process analytical technologies (PAT), designed to assist pharmaceutical developers/ manufacturers in achieving drug commercialisation objectives, whether for scale-up or scale-down.
All aspects of the range are designed for use at R&D scale, but capable of use at full GMP production too.
This allows scientists to know from the outset of an R&D programme that the technology can be scaled up successfully – it is imperative technology can work at the commercial scale, as well as R&D.
Some of the most significant freeze drying data interpretation tools for key data post-processing are: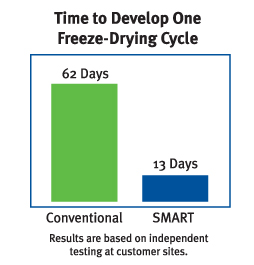
This is a primary drying cycle optimization tool for in depth product information to determine:
-
- The ice interface temperature
- Product resistance (Rp)
- Cycle optimisation
- LyoFlux® TDLAS Sensors
Available for use at any scale for the accurate measurement of vapor mass flow in the calculation of critical product attributes such as:
-
- Primary and secondary endpoints
- Average product temperature & resistance
- Heat transfer coefficients, such as Kv
- Assists with engineering freeze dryer performance qualification
- Tempris® Wireless Sensors
All Line of SightTM freeze dryers are compatible for use with Tempris probes. Wireless probes allow the same probe type to be used for R&D and production, whereas wired probes used for R&D are not compatible at manufacturing scale.
- ControLyo® Nucleation Technology
- Precise control of the freezing point in all vials simultaneously, including vials with a thermocouples
- Increasing nucleation temperature, lowering supercooling, saving time and money
- Larger ice crystals – giving a reduced cycle time and faster reconstitution times
- To discover more about the improvement in cake appearance, reduced protein aggregation, skin formation, and vial cracking, watch this short video – https://youtu.be/JM00HTm8XaU
This freeze dryer enables accelerated freeze drying cycle development with as few as 7 vials and has all of the functionality listed above which is particularly beneficial when small scale development is required due to:
-
- High costs of the product being trialled
- Low availability of the product itself – allowing R&D to occur with very small sample volumes, creating data that can be used efficiently at larger scale
As can be seen from the importance of PAT at the heart of Line of SightTM, whilst the science of freeze drying remains King in the production of viable product, it is apparent that one of the most necessary/ critical elements of processing is the availability of auditable data, which, no matter how good your product looks and performs post-process, generates both regulatory and consumer confidence in the product and subsequently the manufacturer.
To discover more about PAT and Line of SightTM, speak to a Biopharma Group specialist http://bit.ly/BPSContact or see freeze drying equipment via www.bpscrowthorne.ie